-
Brusch JL. Infective endocarditis and its mimics in the critical care unit. In: Cunha BA, ed. Infectious Diseases in Critical Care. 2nd ed. New York, NY: Informa Healthcare; 2007:261-2.
-
Karchmer AW. Infective endocarditis. In: Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. WB Saunders Co; 2005:1633-1658.
-
Karchmer AW. Infective endocarditis. In: Harrison's Principles of Internal Medicine. 16th ed. McGraw-Hill; 2005:731-40.
-
Lerner PI, Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. Feb 17 1966;274(7):388-93 concl. [Medline].
-
Lerner PI, Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. Feb 3 1966;274(5):259-66 contd. [Medline].
-
Lerner PI, Weinstein L. Infective endocarditis in the antibiotic era. N Engl J Med. Jan 27 1966;274(4):199-206 contd. [Medline].
-
Brusch J. Infective Endocarditis: Management in the Era of Intravascular Devices. New York, NY: Informa Healthcare; 2007.
-
Baddour LM, Wilson LM. Infections of prosthetic valves and other cardiovascular devices: intravascular devices. In: Mandell GL, Bennett JE, Dolin R, eds. Mandel, Douglas and Bennett's Principles and Practice and Infectious Diseases. 5th ed. Philadelphia, Pa: Elsevier; 2005:1022-44.
-
Weinstein LW, Brusch JL. Infective Endocarditis. New York, NY: Oxford University Press; 1996.
-
Wang A, Athan E, Pappas PA, Fowler VG Jr, Olaison L, Paré C, et al. Contemporary clinical profile and outcome of prosthetic valve endocarditis. JAMA. Mar 28 2007;297(12):1354-61. [Medline].
-
Miró JM, del Río A, Mestres CA. Infective endocarditis in intravenous drug abusers and HIV-1 infected patients. Infect Dis Clin North Am. Jun 2002;16(2):273-95, vii-viii. [Medline].
-
Xiong YQ, Fowler VG, Yeaman MR, Perdreau-Remington F, Kreiswirth BN, Bayer AS. Phenotypic and genotypic characteristics of persistent methicillin-resistant Staphylococcus aureus bacteremia in vitro and in an experimental endocarditis model. J Infect Dis. Jan 15 2009;199(2):201-8. [Medline]. [Full Text].
-
Chu VH, Miro JM, Hoen B, Cabell CH, Pappas PA, Jones P, et al. Coagulase-negative staphylococcal prosthetic valve endocarditis--a contemporary update based on the International Collaboration on Endocarditis: prospective cohort study. Heart. Apr 2009;95(7):570-6. [Medline].
-
Reyes MP, Ali A, Mendes RE, Biedenbach DJ. Resurgence of Pseudomonas endocarditis in Detroit, 2006-2008. Medicine (Baltimore). Sep 2009;88(5):294-301. [Medline].
-
Murdoch DR, Corey GR, Hoen B, Miró JM, Fowler VG Jr, Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. Mar 9 2009;169(5):463-73. [Medline].
-
Baddley JW, Benjamin DK Jr, Patel M, Miró J, Athan E, Barsic B, et al. Candida infective endocarditis. Eur J Clin Microbiol Infect Dis. Jul 2008;27(7):519-29. [Medline]. [Full Text].
-
Chu VH, Woods CW, Miro JM, Hoen B, Cabell CH, Pappas PA, et al. Emergence of coagulase-negative staphylococci as a cause of native valve endocarditis. Clin Infect Dis. Jan 15 2008;46(2):232-42. [Medline].
-
Hill EE, Herijgers P, Claus P, Vanderschueren S, Herregods MC, Peetermans WE. Infective endocarditis: changing epidemiology and predictors of 6-month mortality: a prospective cohort study. Eur Heart J. Jan 2007;28(2):196-203. [Medline].
-
Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. Nov 1 2001;345(18):1318-30. [Medline].
-
Tleyjeh IM, Steckelberg JM, Murad HS, Anavekar NS, Ghomrawi HM, Mirzoyev Z, et al. Temporal trends in infective endocarditis: a population-based study in Olmsted County, Minnesota. JAMA. Jun 22 2005;293(24):3022-8. [Medline].
-
Mendiratta P, Tilford JM, Prodhan P, Cleves MA, Wei JY. Trends in hospital discharge disposition for elderly patients with infective endocarditis: 1993 to 2003. J Am Geriatr Soc. May 2009;57(5):877-81. [Medline].
-
Wallace SM, Walton BI, Kharbanda RK, Hardy R, Wilson AP, Swanton RH. Mortality from infective endocarditis: clinical predictors of outcome. Heart. Jul 2002;88(1):53-60. [Medline]. [Full Text].
-
Durante-Mangoni E, Bradley S, Selton-Suty C, Tripodi MF, Barsic B, Bouza E, et al. Current features of infective endocarditis in elderly patients: results of the International Collaboration on Endocarditis Prospective Cohort Study. Arch Intern Med. Oct 27 2008;168(19):2095-103. [Medline].
-
Sonneville R, Mirabel M, Hajage D, et al. Neurologic complications and outcomes of infective endocarditis in critically ill patients: The ENDOcardite en REAnimation prospective multicenter study. Crit Care Med. Jun 2011;39(6):1474-1481. [Medline].
-
Chu VH, Cabell CH, Benjamin DK Jr, Kuniholm EF, Fowler VG Jr, Engemann J, et al. Early predictors of in-hospital death in infective endocarditis. Circulation. Apr 13 2004;109(14):1745-9. [Medline].
-
[Guideline] National Institute for Health and Clinical Excellence. Prophylaxis against infective endocarditis. Antimicrobial prophylaxis against infective endocarditis in adults and children undergoing interventional procedures. 2008. (NICE clinical guideline No. 64).
-
[Guideline] Baddour LM, Epstein AE, Erickson CC, et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. Jan 26 2010;121(3):458-77. [Medline].
-
Epaulard O, Roch N, Potton L, Pavese P, Brion JP, Stahl JP. Infective endocarditis-related stroke: diagnostic delay and prognostic factors. Scand J Infect Dis. 2009;41(8):558-62. [Medline].
-
Crawford MH, Durack DT. Clinical presentation of infective endocarditis. Cardiol Clin. May 2003;21(2):159-66, v. [Medline].
-
Di Salvo G, Habib G, Pergola V, Avierinos JF, Philip E, Casalta JP, et al. Echocardiography predicts embolic events in infective endocarditis. J Am Coll Cardiol. Mar 15 2001;37(4):1069-76. [Medline].
-
Eknoyan G, Lister BJ, Kim HS, Greenberg SD. Renal complications of bacterial endocarditis. Am J Nephrol. 1985;5(6):457-69. [Medline].
-
Pruitt AA, Rubin RH, Karchmer AW, Duncan GW. Neurologic complications of bacterial endocarditis. Medicine (Baltimore). Jul 1978;57(4):329-43. [Medline].
-
[Guideline] Sexton DJ, Spelman D. Current best practices and guidelines. Assessment and management of complications in infective endocarditis. Cardiol Clin. May 2003;21(2):273-82, vii-viii. [Medline].
-
Snygg-Martin U, Gustafsson L, Rosengren L, Alsiö A, Ackerholm P, Andersson R, et al. Cerebrovascular complications in patients with left-sided infective endocarditis are common: a prospective study using magnetic resonance imaging and neurochemical brain damage markers. Clin Infect Dis. Jul 1 2008;47(1):23-30. [Medline].
-
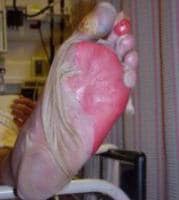
Terpenning MS, Buggy BP, Kauffman CA. Infective endocarditis: clinical features in young and elderly patients. Am J Med. Oct 1987;83(4):626-34. [Medline].
-
Bansal RC. Infective endocarditis. Med Clin North Am. Sep 1995;79(5):1205-40. [Medline].
-
Conlon PJ, Jefferies F, Krigman HR, Corey GR, Sexton DJ, Abramson MA. Predictors of prognosis and risk of acute renal failure in bacterial endocarditis. Clin Nephrol. Feb 1998;49(2):96-101. [Medline].
-
Cosmi JE, Tunick PA, Kronzon I. Mortality in patients with paravalvular abscess diagnosed by transesophageal echocardiography. J Am Soc Echocardiogr. Jul 2004;17(7):766-8. [Medline].
-
Cunha BA, Gill MV, Lazar JM. Acute infective endocarditis. Diagnostic and therapeutic approach. Infect Dis Clin North Am. Dec 1996;10(4):811-34. [Medline].
-
Fowler VG Jr, Scheld WM, Bayer AS. Endocarditis and intravascular infections. In: Mandell GL, Bennett JA, Dolin R, eds. Principles and Practice of Infectious Diseases. 6th ed. Philadelphia, Penn: Elseiver; 2005:975-1021.
-
Roberts NK, Somerville J. Pathological significance of electrocardiographic changes in aortic valve endocarditis. Br Heart J. May 1969;31(3):395-6. [Medline].
-
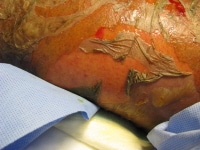
Robinson SL, Saxe JM, Lucas CE, Arbulu A, Ledgerwood AM, Lucas WF. Splenic abscess associated with endocarditis. Surgery. Oct 1992;112(4):781-6; discussion 786-7. [Medline].
-
Weinstein L. Life-threatening complications of infective endocarditis and their management. Arch Intern Med. May 1986;146(5):953-7. [Medline].
-
Weinstein L, Schlesinger JJ. Pathoanatomic, pathophysiologic and clinical correlations in endocarditis (first of two parts). N Engl J Med. Oct 17 1974;291(16):832-7. [Medline].
-
Wang CC, Lee CH, Chan CY, Chen HW. Splenic infarction and abscess complicating infective endocarditis. Am J Emerg Med. Oct 2009;27(8):1021.e3-5. [Medline].
-
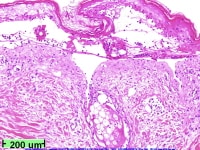
Kradin RL. Pathology of infective endocarditis. In: Brusch JL, ed. Infective Endocarditis: Management in the Era of Intravascular Devices. New York, NY: Informa Healthcare; 2007:101-18.
-
Brusch JL. Pathoanatomical, pathophysiological and clinical correlations. In: Brusch JL, ed. Infective Endocarditis: Management in the Era of Intravascular Devices. New York, NY: Informa Healthcare; 2007:119-41.
-
Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med. Mar 1994;96(3):200-9. [Medline].
-
[Guideline] Towns ML, Reller LB. Diagnostic methods current best practices and guidelines for isolation of bacteria and fungi in infective endocarditis. Infect Dis Clin North Am. Jun 2002;16(2):363-76, ix-x. [Medline].
-
Archibald LK, Pallangyo K, Kazembe P, Reller LB. Blood culture contamination in Tanzania, Malawi, and the United States: a microbiological tale of three cities. J Clin Microbiol. Dec 2006;44(12):4425-9. [Medline]. [Full Text].
-
Bates DW, Goldman L, Lee TH. Contaminant blood cultures and resource utilization. The true consequences of false-positive results. JAMA. Jan 16 1991;265(3):365-9. [Medline].
-
Khatib R, Riederer K, Saeed S, Johnson LB, Fakih MG, Sharma M, et al. Time to positivity in Staphylococcus aureus bacteremia: possible correlation with the source and outcome of infection. Clin Infect Dis. Sep 1 2005;41(5):594-8. [Medline].
-
Lee A, Mirrett S, Reller LB, Weinstein MP. Detection of bloodstream infections in adults: how many blood cultures are needed?. J Clin Microbiol. Nov 2007;45(11):3546-8. [Medline]. [Full Text].
-
[Guideline] Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin Infect Dis. Jul 1 2009;49(1):1-45. [Medline].
-
Weinbaum FI, Lavie S, Danek M, Sixsmith D, Heinrich GF, Mills SS. Doing it right the first time: quality improvement and the contaminant blood culture. J Clin Microbiol. Mar 1997;35(3):563-5. [Medline]. [Full Text].
-
Weinstein MP. Blood culture contamination: persisting problems and partial progress. J Clin Microbiol. Jun 2003;41(6):2275-8. [Medline]. [Full Text].
-
Casella F, Rana B, Casazza G, Bhan A, Kapetanakis S, Omigie J, et al. The potential impact of contemporary transthoracic echocardiography on the management of patients with native valve endocarditis: a comparison with transesophageal echocardiography. Echocardiography. Sep 2009;26(8):900-6. [Medline].
-
Choussat R, Thomas D, Isnard R, Michel PL, Iung B, Hanania G, et al. Perivalvular abscesses associated with endocarditis; clinical features and prognostic factors of overall survival in a series of 233 cases. Perivalvular Abscesses French Multicentre Study. Eur Heart J. Feb 1999;20(3):232-41. [Medline].
-
[Guideline] Cheitlin MD, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davis JL, et al. ACC/AHA/ASE 2003 guideline update for the clinical application of echocardiography: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASE Committee to Update the 1997 Guidelines for the Clinical Application of Echocardiography). Circulation. Sep 2 2003;108(9):1146-62. [Medline].
-
Jassal DS, Picard MH. Echocardiography. In: Brusch JL, ed. Infective Endocarditis: Management in the Era of Intravascular Devices. New York, NY: Informa Healthcare; 2007:255-72.
-
Roe MT, Abramson MA, Li J, Heinle SK, Kisslo J, Corey GR, et al. Clinical information determines the impact of transesophageal echocardiography on the diagnosis of infective endocarditis by the duke criteria. Am Heart J. Jun 2000;139(6):945-51. [Medline].
-
Feuchtner GM, Stolzmann P, Dichtl W, Schertler T, Bonatti J, Scheffel H, et al. Multislice computed tomography in infective endocarditis: comparison with transesophageal echocardiography and intraoperative findings. J Am Coll Cardiol. Feb 3 2009;53(5):436-44. [Medline].
-
Fowler VG Jr, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, Rupp ME, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus. N Engl J Med. Aug 17 2006;355(7):653-65. [Medline].
-
Baddour LM, Wilson WR, Bayer AS, Fowler VG Jr, Bolger AF, Levison ME, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. Jun 14 2005;111(23):e394-434. [Medline].
-
Buckholz K, Larsen CT. Severity of gentamicin is nephrotoxic effect on patients with infective endocarditis: a prospective observation of cohort study of 373 patients. Clinical Infect Dis. 2009;48:65-71.
-
Cunha BA. Persistent S. aureus acute bacteremia: clinical pathway for diagnosis and treatment. Antibiot for Clin. 2006;10:39-46.
-
Falagas ME, Manta KG, Ntziora F, Vardakas KZ. Linezolid for the treatment of patients with endocarditis: a systematic review of the published evidence. J Antimicrob Chemother. Aug 2006;58(2):273-80. [Medline].
-
Jones T, Yeaman MR, Sakoulas G, Yang SJ, Proctor RA, Sahl HG, et al. Failures in clinical treatment of Staphylococcus aureus Infection with daptomycin are associated with alterations in surface charge, membrane phospholipid asymmetry, and drug binding. Antimicrob Agents Chemother. Jan 2008;52(1):269-78. [Medline]. [Full Text].
-
Sakoulas G, Moise-Broder PA, Schentag J, Forrest A, Moellering RC Jr, Eliopoulos GM. Relationship of MIC and bactericidal activity to efficacy of vancomycin for treatment of methicillin-resistant Staphylococcus aureus bacteremia. J Clin Microbiol. Jun 2004;42(6):2398-402. [Medline]. [Full Text].
-
Fowler VG Jr, Li J, Corey GR, Boley J, Marr KA, Gopal AK, et al. Role of echocardiography in evaluation of patients with Staphylococcus aureus bacteremia: experience in 103 patients. J Am Coll Cardiol. Oct 1997;30(4):1072-8. [Medline].
-
Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. Jun 22 2005;293(24):3012-21. [Medline].
-
Khatib R, Johnson LB, Fakih MG, Riederer K, Khosrovaneh A, Shamse Tabriz M, et al. Persistence in Staphylococcus aureus bacteremia: incidence, characteristics of patients and outcome. Scand J Infect Dis. 2006;38(1):7-14. [Medline].
-
Duval X, Selton-Suty C, Alla F, Salvador-Mazenq M, Bernard Y, Weber M, et al. Endocarditis in patients with a permanent pacemaker: a 1-year epidemiological survey on infective endocarditis due to valvular and/or pacemaker infection. Clin Infect Dis. Jul 1 2004;39(1):68-74. [Medline].
-
[Guideline] Olaison L, Pettersson G. Current best practices and guidelines indications for surgical intervention in infective endocarditis. Infect Dis Clin North Am. Jun 2002;16(2):453-75, xi. [Medline].
-
Mekontso Dessap A, Zahar JR, Voiriot G, Ali F, Aissa N, Kirsch M, et al. Influence of preoperative antibiotherapy on valve culture results and outcome of endocarditis requiring surgery. J Infect. Jul 2009;59(1):42-8. [Medline].
-
[Guideline] Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. Oct 9 2007;116(15):1736-54. [Medline].
-
Pronovost P, Needham D, Berenholtz S, Sinopoli D, Chu H, Cosgrove S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. Dec 28 2006;355(26):2725-32. [Medline].
-
Dajani AS, Taubert KA, Wilson W, Bolger AF, Bayer A, Ferrieri P, et al. Prevention of bacterial endocarditis. Recommendations by the American Heart Association. JAMA. Jun 11 1997;277(22):1794-801. [Medline].
-
Bach DS. Perspectives on the American College of Cardiology/American Heart Association guidelines for the prevention of infective endocarditis. J Am Coll Cardiol. May 19 2009;53(20):1852-4. [Medline].
-
Thornhill MH, Dayer MJ, Forde JM, et al. Impact of the NICE guideline recommending cessation of antibiotic prophylaxis for prevention of infective endocarditis: before and after study. BMJ. May 3 2011;342:d2392. [Medline]. [Full Text].
-
Nishimura RA, Carabello BA, Faxon DP, Freed MD, Lytle BW, O'Gara PT, et al. ACC/AHA 2008 guideline update on valvular heart disease: focused update on infective endocarditis: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines: endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. Aug 2008;118(8):887-96.
-
Netzer RO, Altwegg SC, Zollinger E, Täuber M, Carrel T, Seiler C. Infective endocarditis: determinants of long term outcome. Heart. Jul 2002;88(1):61-6. [Medline]. [Full Text].
-
US Food and Drug Administration. FDA Drug Safety Communication: Serious CNS reactions possible when linezolid (Zyvox®) is given to patients taking certain psychiatric medications. Available at http://www.fda.gov/Drugs/DrugSafety/ucm265305.htm. Accessed July 27, 2011.
-
Kiefer T, Park L, Tribouilloy C, Cortes C, Casillo R, Chu V, et al. Association between valvular surgery and mortality among patients with infective endocarditis complicated by heart failure. JAMA. Nov 23 2011;306(20):2239-47. [Medline].